Volcanic eruptions, catastrophic earthquakes, devastating tsunamis, and … acid falling from the sky? Everyone has heard of natural disasters, but one of these things is not like the others. Acid rain is an interesting phenomenon which can be caused by both natural and anthropogenic (human-caused) means.
Rainwater is naturally acidic, but not to any significantly harmful extent, which we will later discuss. However, acid rain occurs when the pH of rainwater drops substantially and begins to cause real damage. But what are the chemical reactions that cause this, and why are they important? Atmospheric chemistry might seem daunting at first glance, but in this article, we will break down the causes, chemical processes, and effects of this phenomenon.
What is the pH Scale?
We must begin by defining our terms. An acid is a substance that can increase the amount of hydrogen ions (H+) in a solution when it is dissolved in water. For this reason, acids are often referred to as proton donors!
A base is something that can accept protons. Perhaps you’ve heard of the pH scale, which is used to measure the acidity or basicity of substances, and is expressed on a range from 0 to 14.
Substances with a pH of 7 are neutral, which means that they are neither acidic nor basic. Substances with a pH above 7.0 are basic, and those below 7.0 are acidic.
Additionally, the pH scale is logarithmic; this means that a solution with a pH of 5 is ten times more acidic than a solution with a pH of 6.0, and so on. To put this in perspective, lemon juice has a pH of 2, while most hand soaps are basic and have a pH of ~9!1
Pure water is neutral, having a pH of 7. However, rainwater is naturally acidic due to its interactions with carbon dioxide (CO2) and other gasses in the atmosphere. Droplets of water within clouds are constantly exposed to CO2, and as a result, some of it dissolves into the water droplets. When this happens, CO2 reacts with water to form carbonic acid (H2CO3).
The formation of this acid slightly lowers the pH of rainwater, resulting in a final average pH of around 5.6.1 While this might sound scary, this reaction is totally natural, and usually does not cause significant environmental harm or damage to human infrastructure.
Further natural acidification of rainwater can occur when other molecules react with atmospheric water vapor. For example, sulfur dioxide (SO2) and hydrogen sulfide (H2S) gasses from volcanic eruptions can dissolve in rainwater. These reactions form even stronger acids such as sulfuric acid (H2SO4) and hydrosulfuric acid (H2SO3).1 Although this can lower the pH of rainwater past its usual point, this does not typically do much ecological harm.
How Does Acid Rain Form?
When people talk about acid rain, they are usually referring to instances when the pH of rainwater has been lowered substantially through anthropogenic means. The pH of acid rain normally falls within a range of 5.0-3.5, but how does this happen, and how are humans contributing to this phenomenon?
One common source of chemicals which lead to anthropogenic acid rain events is coal-burning facilities. People burn coal to create electricity, but this process also releases harmful substances into the atmosphere. When coal is combusted, chemical bonds are broken and release energy, which can be captured and used for human use. Burning coal also releases sulfur, which combines with oxygen in the atmosphere to form SO2.2
Nitric oxides, which are molecules containing nitrogen and one or more atoms of oxygen, are another harmful reactant and driver of acid rain. When fossil fuels are burned at a high enough temperature, such as within the internal combustion engine of an automobile, molecular nitrogen (N2) and oxygen (O2) are able to react to create nitric oxide (NO).
Nitric oxide further reacts with additional oxygen molecules to form nitrogen dioxide (NO2). When nitrogen dioxide is dissolved in water, it forms nitrous acid (HNO2) and nitric acid (HNO3). These stronger acids are eventually rained out of the atmosphere, causing damage to the organisms and environment below.1
Harms Caused By Acid Rain
But why do acids have this corrosive ability, you might ask? Before we answer this question, there is one more term that we must introduce: salt.
Contrary to what many people may believe, salt is not just something that makes french fries taste better. In chemistry, a salt is an ionic compound (a molecule containing both positively and negatively charged atoms) that forms through the reactions of acids and bases. When an acid and base come into contact, they can undergo a neutralization reaction, creating a neutral salt!3
Some materials that we use for construction consist of salts derived from H2CO3. When a strong acid comes into contact with the salt of a weak acid, a complete reaction can take place. Unfortunately, this means that acid rain can degrade structures made of limestone and marble.
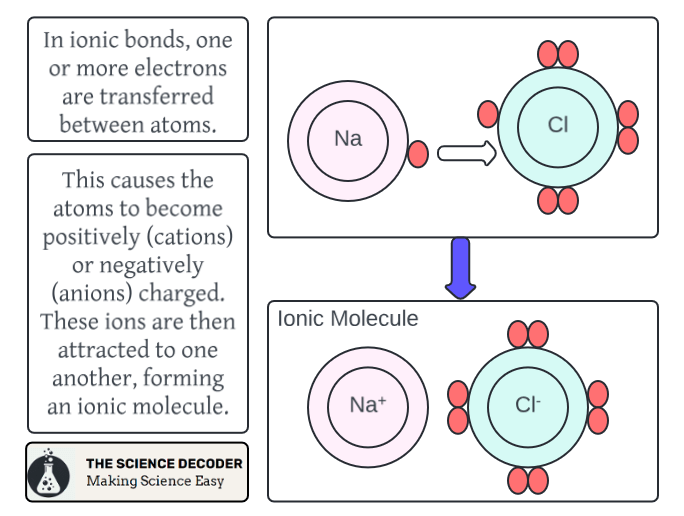
Furthermore, objects made of certain metals undergo oxidation-reduction reactions when they are hit with acid rain. Oxidation-reduction reactions, also known as redox reactions, occur when the oxidation number of a molecule changes.
The oxidation number of a molecular species refers to the amount of electrons it can gain or lose while forming a chemical bond with some other atom.4 Redox reactions are extremely important in living systems, but can cause trouble in the context of acid rain.
When acids come into contact with metals, the hydrogen atoms in acid rain can cause the metal to oxidize, and a redox reaction takes place.5 Over time, these reactions can cause metal structures to degrade and fail, which is especially concerning when we consider the structural integrity of structures like buildings and bridges.
Acid rain can also cause direct harm to living creatures. Organisms have a range of tolerance for many different factors, including pH. If an organism’s environment becomes too acidic or basic, natural processes in the body begin to shut down.
Unfortunately, acid rain can cause entire bodies of water to become acidified, harming whole ecosystems in the process. Amphibious, aquatic plants, microorganisms, and numerous other organisms die off when pH drops below a certain point. For example, the embryos of fish are unable to develop when the pH of water drops below 4.0, which can happen when acid rain is abundant.6
What can we do about the issue of acid rain? Fortunately, the Clean Air Act, passed in 1990, set limitations on the amount of CO2 and SO2 that could be emitted from coal-fired sources. SO2 emissions subsequently dropped by 38.1% from 1995 to 1997, causing acid rain in the United States to decrease.7 This is one example of how political action can create a tangible difference in the chemical makeup of our atmosphere, oceans, and land. In order to avoid future acid rain events (and all of the harms that would accompany them), it is crucial that these policies are maintained.
Sources
(1) 4.8: The Chemistry of Acid Rain. Chemistry LibreTexts. https://chem.libretexts.org/Bookshelves/General_Chemistry/Book%3A_General_Chemistry%3A_Principles_Patterns_and_Applications_(Averill)/04%3A_Reactions_in_Aqueous_Solution/4.08%3A_The_Chemistry_of_Acid_Rain (accessed 2024-06-22).
(2) US EPA, O. What is Acid Rain? https://www.epa.gov/acidrain/what-acid-rain (accessed 2024-06-22).
(3) Acids, Bases and Salts – Definition, Dissociation, Neutralization of Acids, Bases and Salts. BYJUS. https://byjus.com/chemistry/acids-bases-salts/ (accessed 2024-06-22).
(4) 4.10: Oxidation-Reduction Reactions. Chemistry LibreTexts. https://chem.libretexts.org/Bookshelves/General_Chemistry/Book%3A_General_Chemistry%3A_Principles_Patterns_and_Applications_(Averill)/04%3A_Reactions_in_Aqueous_Solution/4.10%3A__Oxidation-Reduction_Reactions (accessed 2024-06-22).
(5) Oxidation-reduction reaction | Definition, Examples, & Facts | Britannica. https://www.britannica.com/science/oxidation-reduction-reaction (accessed 2024-06-22).
(6) Singh, A.; Agrawal, M. Acid Rain and Its Ecological Consequences. 2008.
(7) Lynch, J. A.; Bowersox, V. C.; Grimm, J. W. Acid Rain Reduced in Eastern United States. Environ. Sci. Technol. 2000, 34 (6), 940–949. https://doi.org/10.1021/es9901258.


Leave a comment